How Symbravo Works
MoSEIC™ Meloxicam and Rizatriptan in concert
 Novel MoSEIC™ technology accelerates the absorption of meloxicam, decreasing the Tmax from 4-5 hours to <1 hour while maintaining its long half-life.1,2
Novel MoSEIC™ technology accelerates the absorption of meloxicam, decreasing the Tmax from 4-5 hours to <1 hour while maintaining its long half-life.1,2

formulation of SYMBRAVO,
the absorption of both active
ingredients is accelerated
while meloxicam’s long
half-life is maintained.1,2
 Not actual size.
The mechanism of action for SYMBRAVO is not fully understood. The clinical significance of MoSEIC™ and its pharmacokinetic impact on the active ingredients in SYMBRAVO have not been established.
Not actual size.
The mechanism of action for SYMBRAVO is not fully understood. The clinical significance of MoSEIC™ and its pharmacokinetic impact on the active ingredients in SYMBRAVO have not been established.
(Maxalt)
The mechanism of action for SYMBRAVO is not fully understood. The clinical significance of MoSEIC™ and its pharmacokinetic impact on the active ingredients in SYMBRAVO have not been established.

The same systemic exposures achieved with SYMBRAVO have not been shown with other formulations of meloxicam or rizatriptan.2
How MoSEIC™ rapid-absorption technology works1
MoSEIC™ technology uses cyclodextrin to form a cyclic carrier molecule. The hydrophobic cavity encapsulates a hydrophobic drug. The hydrophilic exterior dissolves easily in water.
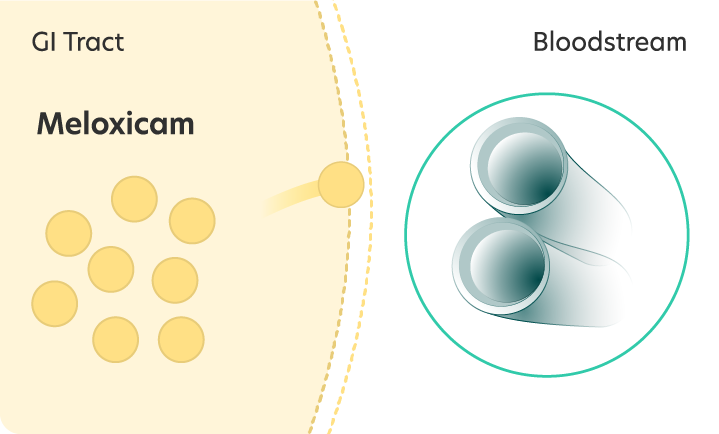
Meloxicam Low solubility and slow absorption
Low solubility and slow absorption

MoSEIC™ Meloxicam Improved solubility and rapid absorption
Improved solubility and rapid absorption
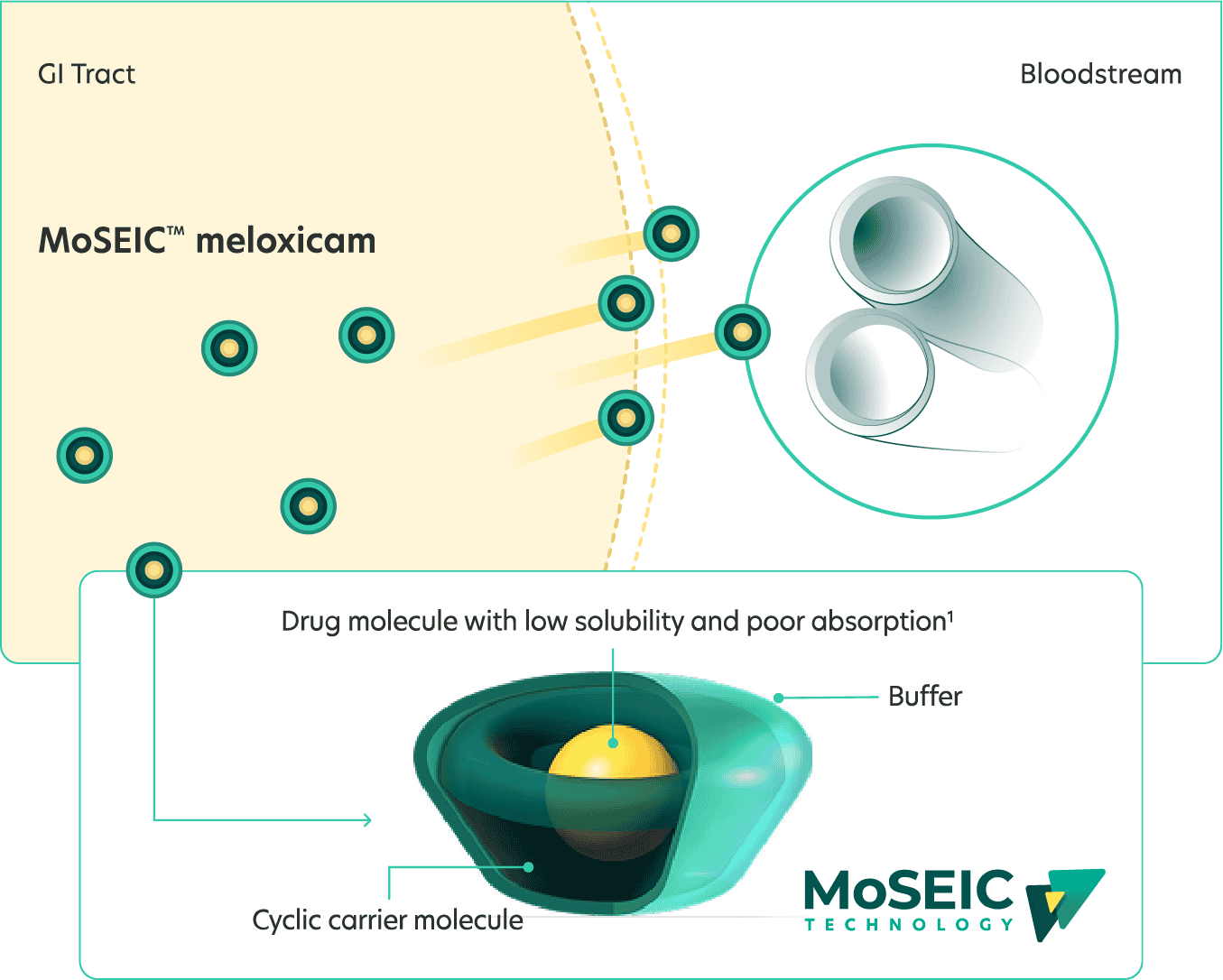
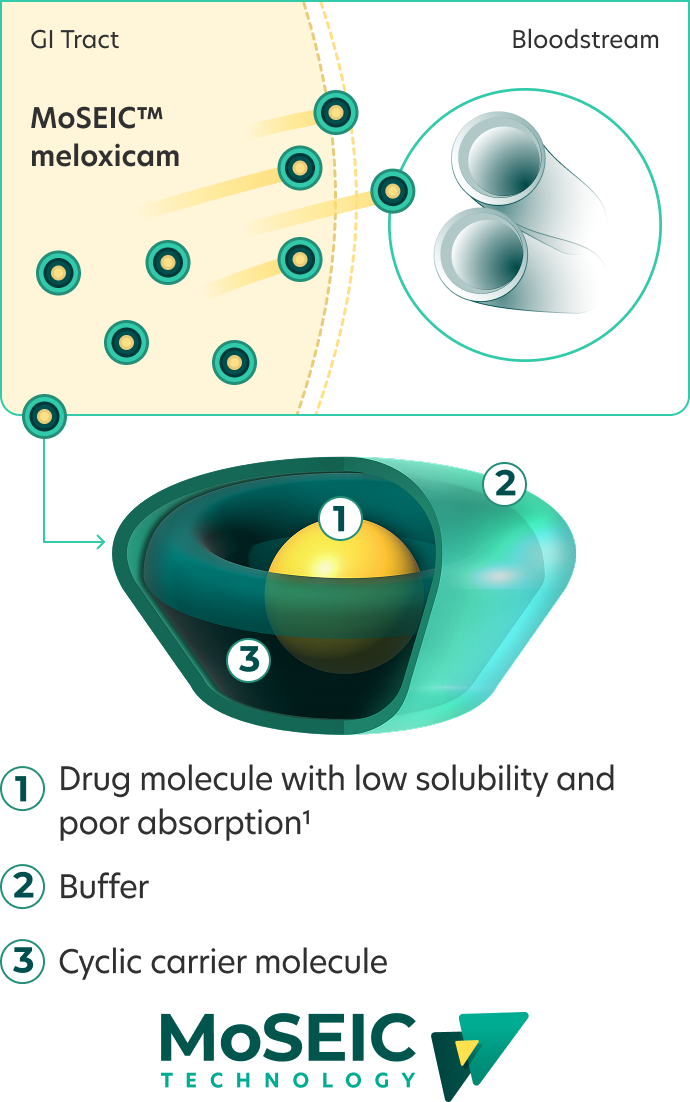
MoSEIC™ Meloxicam: delivered ~4× faster
than oral Meloxicam
tablets1,2,a
Mean Meloxicam concentrations over time for SYMBRAVO vs oral Meloxicam tablets
Tmax <1 hour vs 4-5 hours



MoSEIC™ technology accelerates absorption of meloxicam while maintaining its long half-life of 18 hours.1,2
Up to 2× difference between the absorption rate of
SYMBRAVO Rizatriptan and oral Rizatriptan tablets2,5
SYMBRAVO Rizatriptan absorption vs oral Rizatriptan tablets2,5


SYMBRAVO is thought to address both PGE2 and CGRP—most other medications only address 1 of them2,8‑10
Targeted by Meloxicam1,2,4
Inflammatory mediators (PGE2)
Meloxicam is a COX-1/2 inhibitor with preferential binding affinity for COX-2 (shown in vitro), which inhibits PGE2 synthesis and is thought to reduce neuroinflammation
Targeted by Rizatriptan2,6,7
CGRP-mediated processes
Rizatriptan is an 5-HT1B/1D receptor agonist thought to address multiple neuropeptides including CGRP and CGRP-mediated pain signal transmission.
The mechanism of action for SYMBRAVO is not full understood.
SYMBRAVO delivers the power of a multimechanistic migraine treatment.2
Watch the video to learn more.
- a
- Meloxicam concentrations for SYMBRAVO (20-mg meloxicam/10-mg rizatriptan) and meloxicam concentrations for 15-mg oral meloxicam tablets (Mobic) are from separate studies. Phase 1 PK data trial evaluated healthy subjects in a fasting state.1
- b
- Absorption rate is defined by time to reach peak drug plasma concentrations (Tmax).
- CGRP, calcitonin gene-related peptide; MoSEIC, Molecular Solubility Enhanced Inclusion Complex; NSAID, nonsteroidal anti-inflammatory drug; PGE2, prostaglandin E2.
REFERENCES
- 1. O’Gorman C, Jones A, TenHuisen K, et al. AXS-07 (MoSEIC™ meloxicam/rizatriptan): novel oral therapeutic in clinical development for the acute treatment of migraine. Presented at: 19th International Headache Society Congress, September 5–8, 2019.
- 2. SYMBRAVO [prescribing information]. New York, NY: Axsome Therapeutics, Inc.
- 3. MOBIC [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.
- 4. Schattenkirchner M. Meloxicam: a selective COX-2 inhibitor non-steroidal anti-inflammatory drug. Expert Opin lnvestig Drugs. 1997;6(3):321-334.
- 5. MAXALT [prescribing information]. Jersey City, NJ: Organon Global Inc.
- 6. Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019;18(8):795-804.
- 7. Hargreaves R. Pharmacology and potential mechanisms of action of rizatriptan. Cephalalgia. 2000;20(1_suppl):2-9.
- 8. Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):274-290.
- 9. Rissardo JP, Caprara ALF. Gepants for acute and preventive migraine treatment: a narrative review. Brain Sci. 2022;12(12):1612.
- 10. Altamura C, Brunelli N, Marcosano M, Fofi L, Vernieri F. Gepants -a long way to cure: a narrative review. Neurol Sci. 2022;43(9):5697-5708.
